Seznamy 60 Draw An Atom Of Fluorine Zdarma
Seznamy 60 Draw An Atom Of Fluorine Zdarma. Fluorine is a halogen element. Fluorine has an atomic number of 9. Find the element in the periodic table. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. First you make a circle and inside that you will write neutron =9 …
Prezentováno Solved Consider How Groups On The Aldehydes Can Influence Eaclivity Of The Carbonyl Group 0 4 Draw Bond Polarity Arrow Below Illustrating The Inductive Effect Of The Fluorine Atom How Does The Fluorine Atom
Fluorine has an atomic number of 9. I show you where fluorine is on the periodic table and how to determine. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.
Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

Find the element in the periodic table.. First you make a circle and inside that you will write neutron =9 … I show you where fluorine is on the periodic table and how to determine... This means there are 9 protons in the nucleus.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. Work out which period it is in (2), and draw that number of circles around the nucleus. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Find the element in the periodic table. Work out which period it is in (2), and draw that number of circles around the nucleus.

It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 05.05.2013 · how do you make a fluorine atom?

The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole)... In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Look at the atomic number of fluorine on the periodic table = 9. Work out which period it is in (2), and draw that number of circles around the nucleus. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

Find the element in the periodic table. First you make a circle and inside that you will write neutron =9 … This means there are 9 protons in the nucleus. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine has an atomic number of 9... So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.

05.05.2013 · how do you make a fluorine atom? Look at the atomic number of fluorine on the periodic table = 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The active atomic mass of the fluorine atom is 18.9984. Fluorine is a halogen element. Fluorine is a chemical element with the symbol f and atomic number 9. The active atomic mass of the fluorine atom is 18.9984.

05.05.2013 · how do you make a fluorine atom? . 05.05.2013 · how do you make a fluorine atom?

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. The active atomic mass of the fluorine atom is 18.9984. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine has an atomic number of 9. Fluorine is a halogen element. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Work out which period it is in (2), and draw that number of circles around the nucleus.

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Fluorine is a chemical element with the symbol f and atomic number 9. Find the element in the periodic table. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 The active atomic mass of the fluorine atom is 18.9984. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) I show you where fluorine is on the periodic table and how to determine. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).. Find the element in the periodic table.

I show you where fluorine is on the periodic table and how to determine. I show you where fluorine is on the periodic table and how to determine. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Therefore, two atoms of fluorine form covalent bond with each other. Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is a chemical element with the symbol f and atomic number 9. First you make a circle and inside that you will write neutron =9 … Fluorine has an atomic number of 9. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.. Find the element in the periodic table. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Therefore, two atoms of fluorine form covalent bond with each other... So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.

In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Fluorine has an atomic number of 9. Find the element in the periodic table. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Fluorine has an atomic number of 9. Fluorine is a chemical element with the symbol f and atomic number 9. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Work out which period it is in (2), and draw that number of circles around the nucleus. 05.05.2013 · how do you make a fluorine atom?

Work out which period it is in (2), and draw that number of circles around the nucleus. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Fluorine is a chemical element with the symbol f and atomic number 9. 05.05.2013 · how do you make a fluorine atom?.. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

05.05.2013 · how do you make a fluorine atom? 05.05.2013 · how do you make a fluorine atom? Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The active atomic mass of the fluorine atom is 18.9984. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Look at the atomic number of fluorine on the periodic table = 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.

The active atomic mass of the fluorine atom is 18.9984. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.. First you make a circle and inside that you will write neutron =9 …

I show you where fluorine is on the periodic table and how to determine. The active atomic mass of the fluorine atom is 18.9984. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. This means there are 9 protons in the nucleus. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7)
If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Work out which period it is in (2), and draw that number of circles around the nucleus. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.. Look at the atomic number of fluorine on the periodic table = 9.

N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.. Fluorine has an atomic number of 9. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

Fluorine is a chemical element with the symbol f and atomic number 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. First you make a circle and inside that you will write neutron =9 … Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Look at the atomic number of fluorine on the periodic table = 9.. This means there are 9 protons in the nucleus.

This means there are 9 protons in the nucleus.. Therefore, two atoms of fluorine form covalent bond with each other. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The active atomic mass of the fluorine atom is 18.9984. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. I show you where fluorine is on the periodic table and how to determine. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Fluorine has an atomic number of 9.

4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. First you make a circle and inside that you will write neutron =9 … This means there are 9 protons in the nucleus. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Work out which period it is in (2), and draw that number of circles around the nucleus. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. 05.05.2013 · how do you make a fluorine atom? 05.05.2013 · how do you make a fluorine atom?

Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. This means there are 9 protons in the nucleus. Fluorine is a halogen element. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

Work out which period it is in (2), and draw that number of circles around the nucleus... The active atomic mass of the fluorine atom is 18.9984. Fluorine has an atomic number of 9. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Fluorine is a halogen element. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 .. Therefore, two atoms of fluorine form covalent bond with each other.

First you make a circle and inside that you will write neutron =9 … . Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms.

It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Look at the atomic number of fluorine on the periodic table = 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is a halogen element. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Therefore, two atoms of fluorine form covalent bond with each other. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.

Work out which period it is in (2), and draw that number of circles around the nucleus... Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is a halogen element. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. This means there are 9 protons in the nucleus. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 First you make a circle and inside that you will write neutron =9 … Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).

Look at the atomic number of fluorine on the periodic table = 9.. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Look at the atomic number of fluorine on the periodic table = 9.. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8

4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. This means there are 9 protons in the nucleus. 05.05.2013 · how do you make a fluorine atom? 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Look at the atomic number of fluorine on the periodic table = 9.. 05.05.2013 · how do you make a fluorine atom?

Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9... The active atomic mass of the fluorine atom is 18.9984. Find the element in the periodic table. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). 05.05.2013 · how do you make a fluorine atom?. First you make a circle and inside that you will write neutron =9 …

The active atomic mass of the fluorine atom is 18.9984.. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. The active atomic mass of the fluorine atom is 18.9984. First you make a circle and inside that you will write neutron =9 … The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Fluorine is a chemical element with the symbol f and atomic number 9. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Find the element in the periodic table.. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Fluorine is a halogen element. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Fluorine is a chemical element with the symbol f and atomic number 9.. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

Look at the atomic number of fluorine on the periodic table = 9. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 05.05.2013 · how do you make a fluorine atom? Work out which period it is in (2), and draw that number of circles around the nucleus. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). This means there are 9 protons in the nucleus. I show you where fluorine is on the periodic table and how to determine.. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.

Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.. Find the element in the periodic table. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Fluorine has an atomic number of 9. I show you where fluorine is on the periodic table and how to determine. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Fluorine is a chemical element with the symbol f and atomic number 9. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8

Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 This means there are 9 protons in the nucleus. Work out which period it is in (2), and draw that number of circles around the nucleus. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The active atomic mass of the fluorine atom is 18.9984.. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell.

N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.. Fluorine has an atomic number of 9.

05.05.2013 · how do you make a fluorine atom?. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. This means there are 9 protons in the nucleus. Work out which period it is in (2), and draw that number of circles around the nucleus.

Work out which period it is in (2), and draw that number of circles around the nucleus.. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Therefore, two atoms of fluorine form covalent bond with each other. This means there are 9 protons in the nucleus. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 First you make a circle and inside that you will write neutron =9 … The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.

Therefore, two atoms of fluorine form covalent bond with each other. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Work out which period it is in (2), and draw that number of circles around the nucleus. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 This means there are 9 protons in the nucleus.. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 This means there are 9 protons in the nucleus. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. 05.05.2013 · how do you make a fluorine atom? The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole).

4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Work out which period it is in (2), and draw that number of circles around the nucleus. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine has an atomic number of 9. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell.

Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Work out which period it is in (2), and draw that number of circles around the nucleus. Look at the atomic number of fluorine on the periodic table = 9. Therefore, two atoms of fluorine form covalent bond with each other. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8. Fluorine is a chemical element with the symbol f and atomic number 9.

It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine has an atomic number of 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Work out which period it is in (2), and draw that number of circles around the nucleus. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas... Fluorine has an atomic number of 9.

Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine has an atomic number of 9. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. 05.05.2013 · how do you make a fluorine atom? As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7). Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The active atomic mass of the fluorine atom is 18.9984. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine is a chemical element with the symbol f and atomic number 9. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Fluorine is a halogen element. I show you where fluorine is on the periodic table and how to determine.

Fluorine has an atomic number of 9.. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. First you make a circle and inside that you will write neutron =9 … The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. 05.05.2013 · how do you make a fluorine atom? The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. Fluorine is a chemical element with the symbol f and atomic number 9. Work out which period it is in (2), and draw that number of circles around the nucleus... In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. I show you where fluorine is on the periodic table and how to determine.
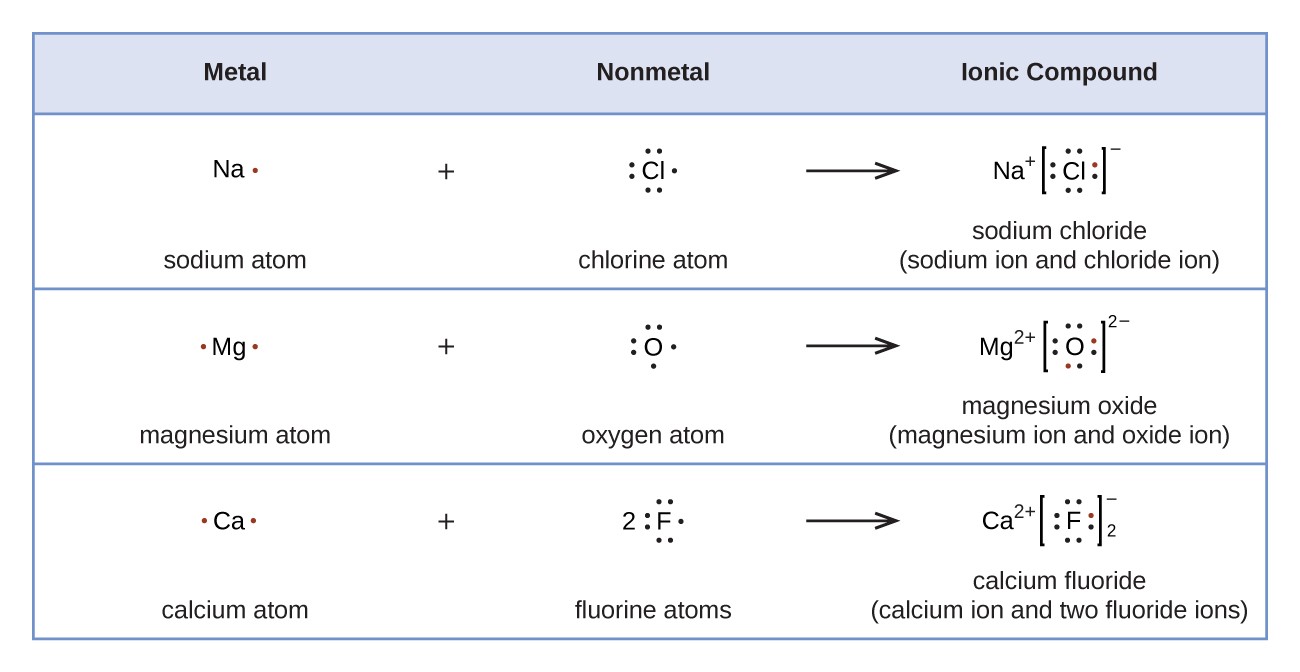
The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell... 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Look at the atomic number of fluorine on the periodic table = 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.
First you make a circle and inside that you will write neutron =9 … The active atomic mass of the fluorine atom is 18.9984. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Look at the atomic number of fluorine on the periodic table = 9. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. I show you where fluorine is on the periodic table and how to determine. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Find the element in the periodic table.

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Find the element in the periodic table. I show you where fluorine is on the periodic table and how to determine. 05.05.2013 · how do you make a fluorine atom? The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). First you make a circle and inside that you will write neutron =9 … Fluorine has an atomic number of 9. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Fluorine is a halogen element.

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) This means there are 9 protons in the nucleus. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Therefore, two atoms of fluorine form covalent bond with each other. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Fluorine is a halogen element. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Fluorine has an atomic number of 9. Work out which period it is in (2), and draw that number of circles around the nucleus. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) The active atomic mass of the fluorine atom is 18.9984. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Look at the atomic number of fluorine on the periodic table = 9.

This means there are 9 protons in the nucleus. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7)

Work out which group the element is in and draw that number of electrons in the outer circle/shell (7). Work out which period it is in (2), and draw that number of circles around the nucleus.

Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is a chemical element with the symbol f and atomic number 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Look at the atomic number of fluorine on the periodic table = 9.. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

Fluorine has an atomic number of 9... The active atomic mass of the fluorine atom is 18.9984. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine is a chemical element with the symbol f and atomic number 9... Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. The active atomic mass of the fluorine atom is 18.9984. Fluorine has an atomic number of 9. Look at the atomic number of fluorine on the periodic table = 9. I show you where fluorine is on the periodic table and how to determine. Therefore, two atoms of fluorine form covalent bond with each other. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven... Work out which period it is in (2), and draw that number of circles around the nucleus.

It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas.. Fluorine is a halogen element.. Fluorine is a halogen element.
Find the element in the periodic table. Fluorine is a chemical element with the symbol f and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. Find the element in the periodic table. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. Therefore, two atoms of fluorine form covalent bond with each other. Fluorine is a halogen element. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine has an atomic number of 9. The active atomic mass of the fluorine atom is 18.9984. First you make a circle and inside that you will write neutron =9 …

This means there are 9 protons in the nucleus. The active atomic mass of the fluorine atom is 18.9984. 05.05.2013 · how do you make a fluorine atom? Find the element in the periodic table. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

Fluorine is a chemical element with the symbol f and atomic number 9... The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Therefore, two atoms of fluorine form covalent bond with each other. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Fluorine has an atomic number of 9. I show you where fluorine is on the periodic table and how to determine. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Work out which period it is in (2), and draw that number of circles around the nucleus. Fluorine is a halogen element. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. Look at the atomic number of fluorine on the periodic table = 9. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell.
It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas... The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. I show you where fluorine is on the periodic table and how to determine. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

First you make a circle and inside that you will write neutron =9 … Look at the atomic number of fluorine on the periodic table = 9. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Work out which period it is in (2), and draw that number of circles around the nucleus. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.. First you make a circle and inside that you will write neutron =9 …

Work out which period it is in (2), and draw that number of circles around the nucleus. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8. Fluorine is a halogen element.

Fluorine is a halogen element... 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. I show you where fluorine is on the periodic table and how to determine. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Find the element in the periodic table. Look at the atomic number of fluorine on the periodic table = 9... Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.

As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Therefore, two atoms of fluorine form covalent bond with each other. Fluorine has an atomic number of 9.

Find the element in the periodic table... 05.05.2013 · how do you make a fluorine atom? Find the element in the periodic table. Therefore, two atoms of fluorine form covalent bond with each other. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. Look at the atomic number of fluorine on the periodic table = 9... So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below.

Fluorine is a halogen element... Fluorine has an atomic number of 9. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. The active atomic mass of the fluorine atom is 18.9984. Look at the atomic number of fluorine on the periodic table = 9. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. Find the element in the periodic table. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). 05.05.2013 · how do you make a fluorine atom?. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.. In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Find the element in the periodic table. The active atomic mass of the fluorine atom is 18.9984. Therefore, two atoms of fluorine form covalent bond with each other. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium.

05.05.2013 · how do you make a fluorine atom?.. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. Find the element in the periodic table. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. This means there are 9 protons in the nucleus. Fluorine has an atomic number of 9. Work out which group the element is in and draw that number of electrons in the outer circle/shell (7) Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.

This means there are 9 protons in the nucleus. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven. The shell closest to the nucleus is called the k shell, next is the l shell, next is the m shell. The active atomic mass of the fluorine atom is 18.9984.. If the first shell can only hold 2 electrons then the second shell must hold 7 electrons.

Fluorine is a halogen element. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Fluorine is neutral and its atomic number is 9, hence, the number of protons and electrons available for its bohr diagram is also 9.
Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. First you make a circle and inside that you will write neutron =9 … In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

I show you where fluorine is on the periodic table and how to determine. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine. Most fluorine around the world has 10 neutrons in the nucleus (mass.figure \(\pageindex{2}\) contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Find the element in the periodic table. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). I show you where fluorine is on the periodic table and how to determine.. 05.05.2013 · how do you make a fluorine atom?

Fluorine is a halogen element. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. Fluorine is a halogen element.. Look at the atomic number of fluorine on the periodic table = 9.

In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom. .. I show you where fluorine is on the periodic table and how to determine.

Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8 Find the element in the periodic table.. N # 8 draw the best lewis symbol for atoms of fluorine and chlorine.

If the first shell can only hold 2 electrons then the second shell must hold 7 electrons. The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option.. Otherwise, always add electrons in pa 2 of 10 questions completed < 03:10 > f7 2 3 4 8

The number of neutrons for the bohr diagram of fluorine can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). 05.05.2013 · how do you make a fluorine atom? In the representation of the electron dot structure of ${{\rm{f}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.

05.05.2013 · how do you make a fluorine atom?. Fluorine is a chemical element with the symbol f and atomic number 9. 4th attempt part 1 (0.5 point) if a lone electron is needed, use the single electron option. So let's draw the electron dot structure of ${{\rm{f}}_{\rm{2}}}$ which is shown below. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Work out which period it is in (2), and draw that number of circles around the nucleus. Therefore, two atoms of fluorine form covalent bond with each other... The valency(valence) of a fluorine atom is 1 and the valence electrons of a fluorine atom are seven.